Building immunity
Advances will help push flu vaccines to more people, more quickly
|
By Dana Goldman
Down a long hallway on the third floor of the Rollins Research Center, the scientists of Richard Compans' lab are hard at work, imagining the unimaginable: A time when patients can self-administer flu vaccines. A time when vaccination does not require exposure to inactive viruses. A time when a universal vaccine could protect from all varieties of influenza: swine, avian, seasonal, and strains still emerging.
But it's not just hope that motivates them as they work. Emory's scientists are fighting the clock against another possible future: a time of pandemic and uncontrollable virus mutation. The recent emergence of H1N1 and H5N1, known colloquially as swine flu and avian flu, have added an even greater sense of urgency to their task.
"The H5N1—the virus derived from avian species—has a 60% mortality," says Emory microbiologist Sang-Moo Kang. Yet that strain of influenza hasn't resulted in many human deaths, because, so far, avian flu spreads only to humans who are in contact with infected birds.
But researchers anticipate that H5N1 might mutate and gain the ability to spread through human-to-human contact. Kang says that would be a devastating game changer. "Once it has come out with some mutation and obtained the capability to transmit among humans, it will be a huge issue. You can imagine how serious it might be," Kang says.
A no-virus vaccine
On a Sunday late last April, Kang's telephone rang. It was his boss, Richard Compans, professor of microbiology and immunology in the medical school. Reports were just coming out about a possible swine flu pandemic. Television news was showing people wearing surgical masks over their faces. The United States was declaring a public health emergency. Compans was getting the first of what would be a blizzard of calls from journalists, all trying to make sense of what was happening.
It was exactly what the laboratory scientists had feared, and it was a situation for which they were prepared.
For eight years, Kang has been working on vaccines at Emory, with steady progress. He and his colleagues have pioneered virus-like particles (VLPs), empty particle shells that mimic the shape of viruses and stimulate the body's immune system to fight back against the perceived threat. Unlike conventional immunizations, VLPs don't actually contain viruses, and their use dramatically limits the possibility of side effects. Also unlike conventional immunizations, they can be produced without using chicken eggs to cultivate the vaccine. "If there's a pandemic and if it derives from the avian species, it may affect the poultry farms that supply the eggs for making vaccines," says Kang. "We can't rely on egg supply."
Just a month before the swine flu outbreak, the lab had published a paper on VLP technology, proving that the particles successfully immunized mice and that immunity wore off more slowly than that induced by conventional flu vaccines in humans. "In a mouse, two years is the average lifespan," Kang says. "We have confirmed that the VLP immune system in mice can last 18 to 20 months. So this protective immunity is maintained for about their lifetime."
In addition, the scientists had discovered another positive about VLPs. Vaccines grown conventionally typically take six months to get to market. Not so with VLPs, says Kang: "After identifying the genes to construct and make the VLPs, we can start to produce vaccines in four to six weeks."
In other words, a burdensome delay in vaccine distribution could be averted if VLP technology worked as well with swine flu as it had with seasonal influenza in mice. "So immediately we contacted the CDC," Kang says. The Georgia Research Alliance awarded the lab and the CDC a grant to test out the VLP vaccine system with the H1N1 virus in animal models. "It's a very good opportunity to prove our alternative vaccine system in this pandemic situation," says Kang.
Patch it
If VLPs do eventually prove useful to people, they will not be alone in changing the landscape of vaccinations. After all, there are both geography and patients' personalities to consider. Some people live far from doctors, and others avoid vaccinations because of fear of side effects or needles. Those populations would be particularly vulnerable in the case of a pandemic.
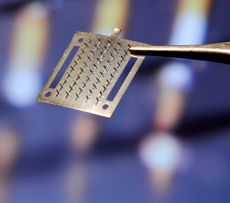
But those patients may soon fear not. Compans' lab has been exploring not just new systems of vaccination but also an alternative to needle-injection vaccination, called microneedle skin patches. The patches have microscopic, barely-visible needles on their surfaces. "We coat the microneedles with inactivated flu virus or flu virus-like particles," says Emory microbiologist Ioanna Skountzou. The patches are temporarily applied to the skin like a bandage, and the vaccine is slowly absorbed through the skin.
In April, Skountzou, Emory colleagues, and collaborators from the Georgia Institute of Technology published a paper in Proceedings of the National Academy of Sciences detailing the value of skin patches. The researchers showed that microneedle skin patches were just as effective as injected vaccines in immunizing mice from seasonal influenza.
The microneedle skin patches could bode well for difficulties with vaccine distribution and for those with needle phobias, says Skountzou. "It can be delivered by mail and can be sent to remote places with no health care providers. It can be tolerated by patients and children with fear of injections," she says.
The skin patches have other advantages as well, says Skountzou. The skin patches are effective with a smaller vaccine dose—called vaccine sparing—than what works in typical injected vaccinations. "Vaccine sparing has several advantages. It can help eliminate side effects caused by the vaccine (skin irritation, swelling, pain), and it is less costly but can still cover the population needs," Skountzou says.
In new ongoing studies, the team is testing the microneedle skin patches on animals like guinea pigs. Skountzou is also combining the microneedle skin patch delivery with adjuvants that further stimulate immune response. She's hoping that adjuvants boost immunity, permit lower doses of vaccine, and drive per-patient costs down even more.
Lower costs, no needles, fewer doctor visits. Less need for vaccine-producing eggs. Skountzou, Kang, and their colleagues have found better ways to prevent illness and contagion. Now, as they hone their vaccination systems, they're also focusing on new implications for their work: If you can give a patient a patch for the seasonal flu, why not also provide a patch for hepatitis or chicken pox? If you can create VLPs mimicking an influenza strain, why not try a VLP to trick the body into fighting other diseases, or all influenza varieties at once?
"We can tackle many diseases and many viruses," says Kang. "It's very exciting." EM