|













|
 |
 |
 Outside
the OR, Csete puts her knowledge of different gases and their potent
effects on living cells to good use when she assumes her other role—that
of a pioneering stem cell researcher. She has turned her expertise
into a crusade of sorts, or at the very least, a research passion.
As head of the medical school’s new core facility for embryonic
stem cell research, she hopes to help turn the promise of stem cell
therapies into reality. Outside
the OR, Csete puts her knowledge of different gases and their potent
effects on living cells to good use when she assumes her other role—that
of a pioneering stem cell researcher. She has turned her expertise
into a crusade of sorts, or at the very least, a research passion.
As head of the medical school’s new core facility for embryonic
stem cell research, she hopes to help turn the promise of stem cell
therapies into reality.
Last fall, she received the go-ahead
to set up the facility, which will supply human embryonic stem cells
grown under tightly controlled conditions to researchers at Emory
and those with the joint Georgia Tech/Emory Center for the Engineering
of Living Tissues (GTEC). Stem cells will not be derived at the
facility. But existing stem cell lines, already approved for use
in federally supported research, will be maintained there. The core
facility will also serve as a resource center, providing technical
assistance and education to investigators conducting various stem
cell research projects. Emory and Georgia Tech are in the process
of purchasing existing cell lines developed at other institutions.
In collaboration with research partners
at the University of Georgia, Csete has also applied for an NIH
grant to fund research on maintaining stem cell lines. UGA scientists
led by Steve Stice have been studying these cells since the NIH
first approved research with existing stem cell lines several years
ago, and Csete’s part of the project would focus on creating the ideal gaseous environment to reduce oxidative stress
and lower rates of cell mutation.
creating the ideal gaseous environment to reduce oxidative stress
and lower rates of cell mutation.
For Csete, the research is more than
a scientific endeavor.
“As physicians, we owe it to
our patients to do this kind of research,” she says. “Most
people think of stem cells as a resource for replacing cells that
have been damaged, which is the Christopher Reeve/Michael J. Fox
approach. But in addition to that, working with stem cells gives
us our first view into the earliest events of human development.
We know tons more about mouse development than we do about human
development. Stem cells also may serve as remarkable tools for screening
drug candidates, modeling diseases, promoting certain kinds of cell
differentiation, and researching protection against cell death.”
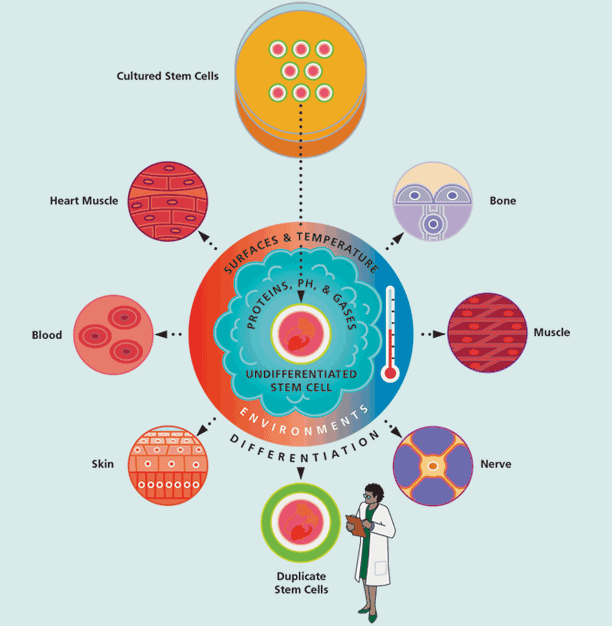
Embryonic stem cells, the primitive,
undifferentiated cells that have the potential to develop into any
kind of cell in the body, have been the subject of heated public
debate because they must be extracted from human embryos. (See
On Point) But glaring headlines aside, the biggest obstacle
blocking new cell-based therapies may be the difficulty of successfully
growing these cells in a laboratory.
Developing an ideal environment is
key to keeping the cells viable, Csete believes, but few researchers
have been interested in this area. Though she has rattled the nerves
of more than a few other scientists with her quest, she hopes her
unique focus will have an important impact on this burgeoning field
of study. |
 |
| |
|
|
 |
| |
|
|
 |
In
1996, Csete interrupted a decades-long career as a liver transplant
anesthesiologist at the University of California-Los Angeles, entering
Caltech as “the world’s oldest graduate student”
to pursue an interest in muscle stem cells, known as “satellite”
cells. She began with the hypothesis that gases have a significant
impact on living cells, no matter where they are. During her four-year
stint in the laboratory, she was shocked to discover that research
biologists were not very conscious of the effects of gases on cell
culture, using everyday room air for their experiments. She knew
that each organ has its own ideal oxygen level, but when she asked
her laboratory colleagues if they knew the body’s normal oxygen
concentration for their organ of interest, she found not a single
faculty member with an answer.
“This told me right away that
my hypothesis was going to be a hard sell,” recalls Csete.
“When I first started asking the question, everyone was very
upset because they were concerned that their tissue-culture work
going back for decades might not be quite right.”
At the time, only two areas of biology
took gases into consideration. Vascular biologists knew that when
an organ’s oxygen supply is lowered to dangerous levels, vascular
cells take up the slack by proliferating and making new blood vessels
to deliver more oxygen. Models of vascular biology have for decades
included very low oxygen conditions. Cancer biologists know that
as tumors outgrow their blood supply, the center of the tumor becomes
very low in oxygen, a condition called hypoxia. The cells that survive
hypoxia become the most dangerously resistant to chemotherapy and
radiation. In some ways, says Csete, these hypoxia-regulated cells
turn into cancer stem cells.
Armed with a PhD from Caltech, Csete
then moved to the University of Michigan, where she continued her
work with oxygen and cell culture, using a specially designed work
area where gases could be controlled during long-term stem cell
growth. She began to ask the simple question of what happens to
stem cells when you change
the gases around them to reflect not room air, but the exact gas
concentration cells actually encounter in the body. She was not
surprised to discover that a particular balance of gases made a
tremendous difference to growing cells. |
 |
| |
|
|
 |
| |
|
|
 |
“We
found that cells are happier and healthier and that they live longer
in oxygen concentrations that reflect the level in the body of 2%
to 5%,” Csete explains. “Maintaining concentrations
in this range is tricky, especially over long periods of time, but
it’s a very powerful tool. I would say with conviction that
based on the kinds of changes we see with different oxygen levels,
you have to be very rigorous and never let in room air.”
Stem cells are very similar to cancer
cells in their tendency to proliferate and migrate, Csete explains,
which is why it is critical to eliminate mutations while growing
the cells. Embryonic stem cells are particularly vulnerable to chromosomal
breaks and severe mutations. Although cells with damaged chromosomes
are naturally programmed to die, those few that manage to survive
would have an enormous potential to reproduce in a tumor-like fashion.
“That’s why, at this point,
you would not consider putting a completely undifferentiated stem
cell into a human until there are better ways of predicting how
to control their properties. And any changes in the growing environment
of embryonic stem cells will lead them to differentiate, so conditions
must be maintained very carefully.”
The stem cell core facility consists
of just two tiny laboratories on campus. But the small space embodies
Csete’s zealous belief in keeping the cells protected from
room air. The cells here will be stored and cultivated in protective
mini-incubators and exposed to very low levels of oxygen ranging
from 1% to 8%. Csete’s prior research showed that under oxygen
conditions that mimic conditions inside the body, stem cells can
be protected from undergoing chromosomal damage.
“There is nothing more fundamental
in biology than oxygen,” Csete emphasizes. “As anesthesiologists,
we’re conscious right up front of what gases do. We monitor
them in the operating room. But for most biologists, the gas context
is just not there. It’s not a major part of what they are
taught. Students who jump right into molecular biology to study
how genes interact inside cells don’t get the bigger picture
that using room air for experiments is aphysiologic.” Csete
says. “There is a huge bank of historical literature from
scientists who have grown cells in room air because of convenience,
and that practice is hard to change because there are thousands
of papers already written based on that method. The vast majority
of laboratories still have incubators that rely on room air, where
the oxygen concentration is 20%.”
Emory and Georgia Tech researchers
are uniquely qualified to study the interaction of embryonic stem
cells with the environment, says Csete.
“Our research—which includes
novel approaches to studying mechanical influences on the cells
and also the effects of signaling from the extracellular matrix
and the effect of the gases—is very likely to lead to a better
understanding of the therapeutic roles of human embryonic stem cells
for a wide variety of diseases.”
Holly Korschun is
the director of science communications at the
Woodruff Health Sciences Center. |
 |
| |
|
|
| |
|
|
 |
| |
 |
|
 |
Stem
Cells 101: A Primer |
 |
 |
by
Marie Csete |
 |
| |
 |
|
 |
Rarely
have science, politics, and medicine collided in
the public imagination as they have over the stem cell debate.
Yet despite all the air and press time, the average person
still has only a vague idea about what these cells are and
where they come from.
First, the basic term “stem
cell” is used to describe a number of types of undifferentiated
cells that have the potential to become different kinds of
specialized cells. Stem cells function as a kind of repair
system for the body. By themselves, they lack the potential
to function physiologically, that is, to carry out specific
bodily tasks. For example, stem cells cannot act as neurons
because they do not express the signaling chemicals that neurons
use to communicate. They cannot act as muscle cells because
they do not express the contractile proteins necessary for
muscle activity.
They have the ability, however,
to make cells (called daughter cells) of more than one type.
For example, certain blood stem cells can generate platelets,
white cells, and red blood cells. Scientists call this ability
to generate diverse cell types multipotentiality.
Potency in stem-cell language refers to a
cell’s potential to  generate
different specialized cell types. Multipotent
stem cells can generate just a few different kinds of cells.
Pluripotent stem cells can generate many
different kinds of specialized cells. Totipotent
stem cells have the potential to generate any cell type in
the body, including germline cells (sperm and egg). Differentiation
is the process by which stem cells mature and change into
physiologically functional, specialized cells. generate
different specialized cell types. Multipotent
stem cells can generate just a few different kinds of cells.
Pluripotent stem cells can generate many
different kinds of specialized cells. Totipotent
stem cells have the potential to generate any cell type in
the body, including germline cells (sperm and egg). Differentiation
is the process by which stem cells mature and change into
physiologically functional, specialized cells.
While multipotent and pluripotent
stem cells exist in the human body in varying degrees throughout
a person’s lifetime, totipotent cells
are found only in embryos in the earliest stages of development.
The totipotency of embryonic stem cells is the reason for
scientific excitement about these cells, but the totipotency
also means that the control of differentiation toward just
a single type of specialized cell is technically difficult,
because all the other options for differentiation have to
be negatively controlled.
Stem cells also have the ability
to self-renew, a property that is unique
to them. When stem cells divide, the new cells can be either
new stem cells or more specialized types of functional cells.
In some cases, stem cells also may undergo asymmetric
division, in which one cell retains its stem cell-ness
and the other goes on to develop into a specialized cell.
People are often confused by
references in the media to “adult” and “embryonic”
stem cells.
Adult stem cells are derived
from tissue sources that have already differentiated. “Adult”
here does not mean from a person who is past puberty. Adult
stem cells can be derived from fetal sources, young people,
or adults.
Embryonic stem cells are derived
from a single cell removed from a fertilized egg that has
been maintained in a lab and allowed to divide to a particular
embryonic stage called the blastocyst. Embryonic stem cells
from the lines approved for research using federal funds come
originally from blastocysts left over from in vitro fertilization
procedures designed to help infertile couples have children.
Only embryonic stem cells are
considered totipotent, whereas adult stem cells are more restricted
in the kinds of specialized cells they can generate. In general,
stem cells are harder to find and grow when derived from older
organs and tissues, and they generally lose some of their
ability to be stem cells with long times in the body. The
aging of stem cells is the focus of work in our lab.
Researchers still have much
to learn about stem cells and how they function, including
how stem cells might be manipulated to treat many different
illnesses and medical conditions. But many scientists believe
these cells hold the potential for great medical and scientific
advances.
If you want to know more about
stem cells, the National Institutes of Health maintains a
website devoted to stem cell research. It can be found on
the web at this address: http://stemcells.nih.gov/info/basics.
Marie Csete is
assistant professor of cell biology and the John E. Steinhaus
Professor of Anesthesiology in the School of Medicine. |
 |
| |

|
|
|
 |
| |
|
|
| |
|
|
| |
|
|
|
|
|

