Moving the HIV Vaccine Forward
By Martha Nolan McKenzie
In 1953, Jonas Salk announced that he had successfully tested the world's first vaccine for polio. Just the year before, there were 58,000 new cases of polio in the United States alone.
Today polio barely warrants attention, thanks to its vaccine. Emory researchers would like to do the same for the human immunodeficiency virus (HIV).
|
More than 1.8 million children took part in Jonas Salk's trial to test his polio vaccine. |
There is no known cure for HIV, but what if you could give a vaccine to protect your patients against HIV, just as you do for mumps or polio? Microbiologist and immunologist Harriet Robinson says that contrary to public perception, real progress is being made toward an HIV vaccine. Robinson, a former professor at Emory, now is full-time at GeoVax, a company she co-founded to commercialize the vaccine developed in her laboratory at Emory, the NIH, and the CDC. GeoVax's first-generation vaccine controlled HIV should a person become infected, but the second generation, which started human trials in May, goes a step further.
"The first generation of our vaccine was primarily controlling infection," says Robinson. "If an animal was vaccinated and then exposed to SIV (simian immunodeficiency virus, the nonhuman primate version of HIV), the vaccine kept the infection to low levels, but it didn't actually prevent infections. With this new vaccine, we are preventing infection in animal models. That is what is exciting."
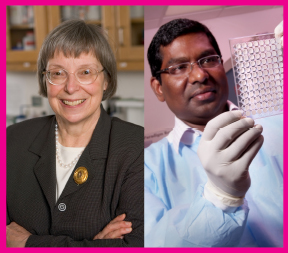
Robinson began working on HIV vaccines in 1992. She was one of the discoverers of DNA vaccines and had been working with a chicken retrovirus as the AIDS epidemic spread. "It was just logical to take this new method of vaccinations and begin to develop it for HIV," says Robinson. Emory microbiologist and immunologist Rama Rao Amara joined her lab in 1999 and conducted much of the original nonhuman primate research. Meanwhile, Robinson formed GeoVax to handle vaccine manufacturing, regulatory filings, and commercialization while Amara continued to oversee the preclinical phase of the vaccine development.
|
Harriet Robinson is testing a vaccine with an added protein that stimulates immune responses. Rama Amara conducted much of the nonhuman primate research for the vaccine. |
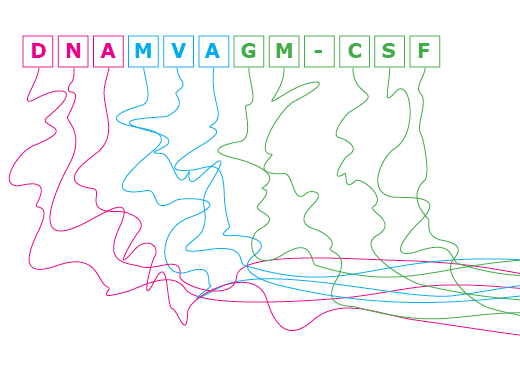
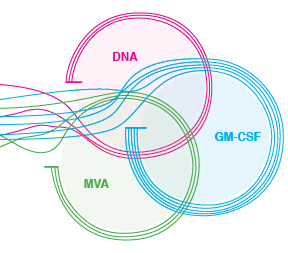
The original vaccine regimen consisted of two inoculations of DNA that contain noninfectious HIV particles to prime the immune response, followed by two inoculations of MVA, an attenuated smallpox vaccine. "DNA is relatively inefficient in entering cells, so we use it to get the immune response primed," says Robinson. "MVA gets into cells more efficiently and produces more protein, so we boost the response with MVA, into which we have tucked HIV genes.
The new vaccine, developed with Amara, keeps the DNA and MVA components but adds the adjuvant GM-CSF (granulocyte-macrophage colony-stimulating factor) in the HIV proteins in the DNA. "GM-CSF is a normal protein that improves immune responses," says Robinson. "It's already being used in a prostate cancer vaccine, and it's also used to stimulate the production of white blood cells in chemotherapy, so it has a good safety record in humans."
In the preclinical trials of the GM-CSF vaccine, monkeys were given two DNA inoculations at months 0 and 2 to prime the vaccine response and then two booster MVA inoculations at months 4 and 6. Six months after the last vaccination, both vaccinated and unvaccinated animals were exposed to SIV once a week for 12 weeks.
The level of virus to which they were exposed, however, was much more potent than would naturally occur.
"In humans, the transmission of HIV is very poor," says Amara. "Only one out of 100 to 500 exposures results in infection. We can't work with such low rates in the lab and get any meaningful results, so we use much higher doses of the virus."
 |
After 12 exposures to SIV, 25% of the animals vaccinated with the original vaccine were protected. However an impressive 70% of the monkeys given the GM-CSF vaccine remained infection free; that translates into a per-exposure vaccine efficacy of 90%. In this and subsequent trials, all of the unvaccinated monkeys became infected.
"Repeated exposures in animals are used to mimic sexual transmission," says Robinson. "The hope is that the results in the nonhuman primate models will translate into vaccine-induced prevention in humans."
The animals that were not infected received a booster MVA dose a year later. Six months later they were exposed to another series of 12 weekly virus challenges. This time four of the five monkeys given the GM-CSF vaccine were protected, and, again, all of the unvaccinated animals were infected.
"No one doing this type of study has ever had enough protected animals to go through a second series of challenges," says Robinson. "These results are very good. The second trial translated into a per-exposure vaccine efficacy rate of 94%."
A third series of exposures was undertaken six months later with a highly neutralization-resistant form of SIV. In this trial, all of the monkeys became infected but not right away. One was infected at the third challenge, one at the 10th, another at the 11th, and the last one at the 12th. "The vaccine was able to significantly delay infection, even against this strain," says Amara. "That is very promising, especially considering the high doses of the virus we are using."
Robinson credits three factors for the vaccine's success. "Of the researchers working on HIV vaccines, we are among the few who designed a vaccine to make a noninfectious virus-like particle—others use mixtures of individual proteins," she says. "We are the only ones who express the full length of the virus envelope. The envelope is the part of the virus that is on the surface that binds to the receptors on T cells. It's what gets the virus into cells, so it is the target for antibodies that can block infections. Other researchers are expressing subunits of the envelope, but we try to mimic the entire form that is on the virus.
"And finally, we are the only ones who are co-expressing GM-CSF in the vaccine," Robinson says. "Others have tried using GM-CSF as an added protein, but we tucked it right into the DNA, so it is expressed off the same DNA that expresses noninfectious virus particles. It's exactly where it needs to be."
Spurred by the promising results in nonhuman primates, clinicians have begun testing the DNA/MVA GM-CSF vaccine in a phase 1 human trial through the HIV Vaccine Trial Network. "We will do a dose escalation, and if this is safe and we show the anticipated immune response, we hope to go directly to a phase 2a/2b trial, which would be a 4,000-person efficacy trial," says Robinson.
While the phase 1 trial is limited to a low-risk population, the phase 2 trial would target a high-risk group. "Phase 2 will test men who have sex with men in the Americas," says Robinson. "We will give half a placebo and half the vaccine and follow them for 1½ years. This will test if the vaccine actually prevents infection in humans."
Robinson also has an ongoing therapeutic trial that tests the vaccine on people already infected with HIV to see if it can control the virus in the absence of drugs.
The impact an effective HIV vaccine could have is hard to overstate. "The United States has a staggering 55,000 new HIV infections each year—that's as many people as died in the Vietnam War," says Robinson. "But only about 35% of those infected have infections that are well controlled by drugs. So the need just in the United States is tremendous, and when you consider the rest of the world, it's incalculable.
"I don't think I appreciated how far we had to go when we started our campaign to make an AIDS vaccine," she says. "I can remember the then-director of Yerkes saying to me in 1998 that, ‘We were launched.' But in actuality we were just starting. Now, 14 years later, people are amazed at how far we have gotten. Right now we are in one of the hardest phases to fund—beyond basic research that can be funded by NIH grants and before the results of efficacy trials. But we have a solid base, and the trick now is to swing for the next highest bar." EM