














|
 |
| |
|
|
 |
 E-mail to a friend
E-mail to a friend  Printer friendly
Printer friendly |
 |
| |
|
|
 |
Rafi Ahmed is curious. Why
does the immune system sometimes fail to eliminate
viruses that cause chronic diseases? Why have vaccines designed
to boost immune response in people with chronic diseases been
so unsuccessful? What he has discovered so far is that once-good
memory T cells in charge of remembering and combating pathogens
can become exhausted and ineffective, allowing viruses to multiply
virtually unchecked.
A multi-institutional team of researchers
headed by Ahmed, a Georgia Research Alliance Eminent Scholar and
director of the Emory Vaccine Center, has identified the exhaustion
pathway and developed a new strategy to re-energize exhausted
T cells. Published in Nature, the strategy helps T cells fight
aback against the once free-swinging viral infections.
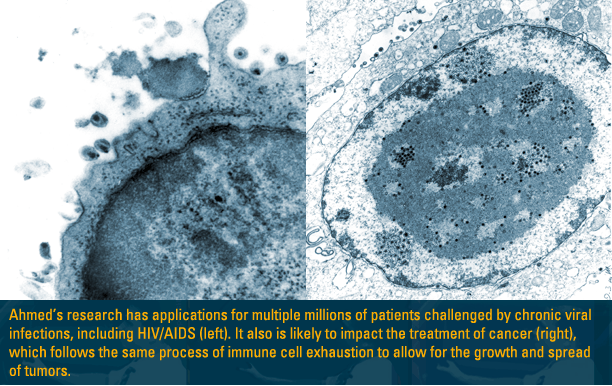
The landmark research has applications
for multiple millions of patients challenged by chronic viral
infections, including HIV/AIDS and hepatitis B and C, and the
strategy also is likely to impact the treatment of cancer, which
follows the same process of immune cell exhaustion to allow for
the growth and spread of tumors. |
 |
| |
|
|
 |
Tired
and worn out |
|
 |
When
first confronted by an acute viral infection, the immune system’s
T cells are full of pep and vigor, proliferating rapidly, secreting
cytokines, killing infected cells, and decreasing the viral load.
Even after the pathogen is eliminated, a group of these cells
remain as highly functional memory cells, ready to mobilize quickly
should the conquered virus reappear.
The same is true in a chronic viral
infection, with T cells starting out strong. However, as the virus
holds on, the cells start to flag. Eventually, they become too
exhausted—“exhausted” serving as the scientific
as well as descriptive term—to function well. Without effective
T cells, the immune system is unable to eliminate the pathogen.
Until the study by Ahmed and his
team, no one understood why the immune cells should become exhausted
in one type of battle and not in another. The researchers tracked
down the essential difference between “good,” or highly
functional T cells, and “bad,” or exhausted ones.
Using a mouse virus that has both acute and chronic strains, they
performed a genome-wide analysis of the genes expressed by exhausted
T cells overcome by the challenge of the chronic strain. They
compared those with genes expressed by functional memory T cells.
The exhausted T cells expressed high levels of an inhibitory receptor.
The functional memory T cells had no detectable level of this
receptor.
No wonder the T cells are exhausted
in chronic infections, says Ahmed. It is as if a driver kept pressing
down on the accelerator of a car, not realizing the emergency
brake was on.
Although scientists in Japan first
discovered the presence of the inhibitory receptor, Ahmed’s
group was the first to show its role in T cell exhaustion. More
important, they also demonstrated how the braking effect of the
inhibitory receptor signals could be blocked with antibodies.
The team used mice that were infected with the chronic strain
of the virus and genetically engineered to be immunosuppressed.
Despite the mice’s crippled immune systems, when they were
given antibodies that blocked the expression of the inhibitory
receptors, their T cells’ ability to function against the
infection was significantly restored. Whereas the virus curbed
the body’s natural immune defenses, the blockade treatment
revved it up again. |
 |
| |
|
|
 |
Applications
in the developing world |
|
 |
Ahmed’s
group now will apply the same immunologic strategy to nonhuman
primates, focusing on hepatitis C. Hepatitis C is the most common
blood-borne viral disease in the United States, affecting more
than 2.5 million Americans and a staggering 170 million people
worldwide. Without effective treatment, many of those infected
develop cirrhosis or liver cancer. The best treatment regimens
now available are effective in only half the cases, and they are
so difficult, expensive, and laden with serious side effects as
to essentially preclude their use in the developing world.
The health and economic impact of
the hepatitis C challenge and the promise of this new immunologic
blockade strategy recently won Ahmed’s research team a $12.5
million grant from the Grand Challenges in Global Health Initiative.
Funded by the Bill & Melinda Gates Foundation, the initiative
seeks to achieve scientific breakthroughs against diseases that
kill millions of people each year in the world’s poorest
countries. Headed by Ahmed, the research project includes collaborators
at Harvard, Columbus Children’s Research Institute, Rockefeller
University, and the NIH. If all goes well, Ahmed expects clinical
trials of a blockade-type vaccine to begin within the next five
to 10 years for those with hepatitis C and other chronic infections
and potentially for patients with certain forms of deadly cancer.
Ahmed hypothesizes that the blockade
vaccine could enhance the effect of other vaccines given therapeutically.
“The combination of the two could be highly synergistic
in both chronic disease and cancer,” he says. |
 |
| |
|
|
 |
| |
|
|
 |
Closing
a hole in the nation's containment strategies |
|
 |
Whereas
Ahmed’s research seeks to amplify the body’s natural
immunity, his long-time collaborator, Christian Larsen, is working
at the other end of the spectrum. Larsen, the Carlos and Marguerite
Mason Professor of Surgery and director of the Emory Transplant
Center, seeks to selectively tamp the immune response to allow
continued tolerance of a transplanted organ while maintaining
ability to fight infection. He is asking a different set of questions
about immune memory. Does a transplant recipient’s immune
system “remember” earlier vaccinations, for example,
against tetanus, measles, or smallpox? Do suppressed immune systems
respond to new vaccinations differently from immune systems that
are intact?
Ahmed and Larsen have been collaborating
on protective immunity for almost a decade now, a partnership
so productive that the NIH has repeatedly called for relationship
advice to pass on to other, less amicably functioning research
teams. This past year, as part of a federal biodefense effort,
the NIH asked for a different kind of advice. Because transplant
patients are severely immunosuppressed, NIH leaders figured that
what Larsen and Ahmed are learning about immune memory in this
population will apply many times over to those with immune systems
partially suppressed by chronic viral or autoimmune diseases or
by chemotherapy. The NIH wants to know how to protect these rapidly
expanding groups of patients from the triple threats of bioterrorism,
emerging infectious diseases, and the ever-present possibility
of a new influenza pandemic.

That leads to another question:
how to protect the whole population from new risks that result
from a continually expanding number of people with compromised
immune systems in its midst. Immunosuppressed people represent
a sizeable hole in any national and global containment strategies
against bioterrorism or threats from emerging infectious disease,
says Larsen. First, they are unable to take some live attenuated
vaccines—smallpox, for example—and when given vaccinations
against other diseases, such as flu, they may be less likely to
develop clinically significant levels of protective antibodies.
Second, they may even intensify the spread of infectious disease,
as the 2003 SARS outbreak demonstrated. The patient known as the
“Toronto super-spreader,” whose extraordinarily high
levels of the SARS virus caused infection among health care workers
despite all precautions, was a lung transplant recipient.
Larsen and Ahmed want to change
this double whammy. As part of a $10 million grant from NIH, they
are mapping the intricate twists and turns of how immunosuppression
changes the response to vaccinations against influenza and smallpox.
They also want to determine whether using new immunosuppressive
drugs can improve that response.
Kidney transplant patients—the
largest transplant population—have been quick to volunteer
for the study measuring immune response following a standard flu
shot. (As part of the control group, Larsen pulls up his own sleeve
for blood tests on a regular basis.) Transplant patients know
that even if they’ve been vaccinated, they get sicker and
are more likely to die of flu. Having the flu also raises the
risk of organ rejection.
Thanks to the availability of new
tools developed by Ahmed and others, researchers have what they
need to describe the magnitude, character, and molecular signature
of the full spectrum of immune responses following vaccination
against flu: the antibodies that neutralize invading viruses;
flu-specific T cells that screen infected cells in the body and
destroy them before they can replicate; and memory T cells that
remain vigilant for any appearance of the virus seen in the vaccine.
The current study will determine
if altering the drug regimen inducing immunosuppression can strengthen
transplant recipients’ response to the flu vaccine (good)
without strengthening recognition and response to the transplanted
kidney (very bad). Currently, scientists do not know what effect
different immunosuppressive drugs have on immunologic memory,
although animal studies have suggested that some drugs cause more
memory decay than others.

The team is making similar comparisons
of immune response to a new form of smallpox vaccine in rhesus
macaques, some immunologically healthy, others taking one of the
immunosuppressant drugs given to humans after transplant. When
smallpox was last active, mortality among the general population
infected with the highly contagious virus was approximately 30%.
The general assumption among scientists and clinicians is that,
should smallpox reappear today, mortality among infected immunosuppressed
patients would approach 100%.
Currently, available smallpox vaccines
use live vaccinia virus. This vaccine works well, effectively
establishing a pool of virus-specific memory T cells that slowly
decline over several decades and B cell populations that remain
stable even longer. But that’s the scenario only in people
with reasonably functioning immune systems. Some people who were
later found to have defects in T cell immunity have had life-threatening
complications to this vaccine. Such findings mean that smallpox
vaccination is contraindicated for immunosuppressed patients,
at least with the current vaccine.
However, a different vaccine, known
as modified vaccinia Ankara strain (MVA), has been created by
passing the live vaccinia virus through 500 generations of chick
embryos, during which time the virus mutates and loses its capacity
to replicate effectively in human cells. Larsen and others now
are studying the immune response to the MVA vaccine in immunosuppressed
monkeys at Yerkes Primate Research Center, research that may well
be applicable to human patients. Does the new vaccine protect
against smallpox infection in these monkeys? Is it safe, or does
it trigger organ rejection? Do monkeys taking one of the new immunosuppressant
drugs have a better immune response? |
 |
| |
|
|
 |
| |
|
|
 |
Mapping,
then tailoring the immune response |
|
 |
The
questions raised in these studies go straight to the heart of
how the immune system works in health and when challenged by chronic
infectious diseases or immune suppression. The devil is in the
details, of course, but once researchers complete the precise
roadmap of immune response over time, the way to change those
details to create a desired immune response will be clearer. Whether
they are restoring memory to T cells long tired of fighting hepatitis
C or teaching the immune system to selectively “forget”
about a transplanted organ, Ahmed and Larsen each hope to help
the other find the keys to perfect immune balance. |
 |
| |
|
|
| |
|
|
|
|
|





